Calcium Carbonate Reacts With Hydrochloric Acid
Once these new bonds are formed the acid and the carbonate no longer. When an acid reacts with a metal carbonate a salt carbon dioxide and water are formed.

How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube
What are the products of this reaction.
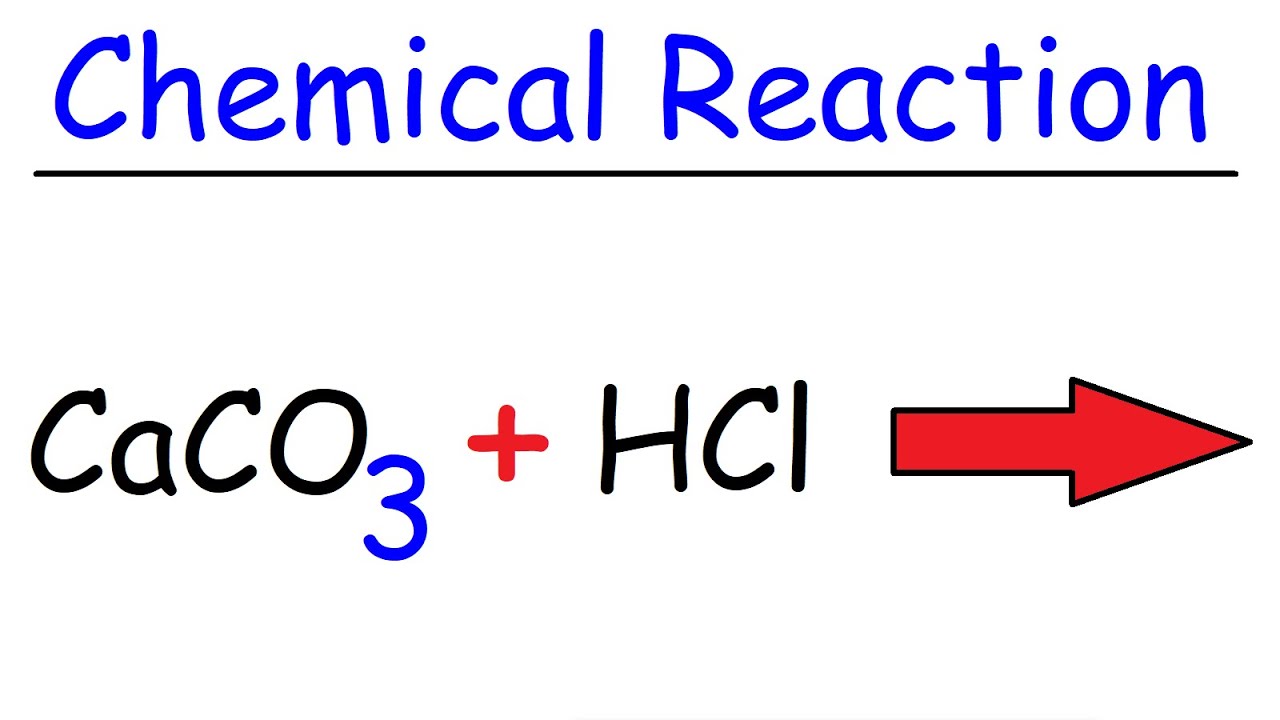
. How many grams of calcium carbonate are required for complete reaction with 350 mL. The balanced reaction is shown below. One way of following the rate of reaction at.
Nitric acid reacts with sodium carbonate to form. According to the balanced chemical equation 2 moles of HCl reacts with 1 mole of CaCO3. In an experiment a learner adds 500 cm3 hydrochloric acid HCℓ with a concentration of 036 moldm-3 to 12 g of magnesium in a test tube.
Chemistry Chemical Reactions Double. CaCO 3 is a base HCl is an acid. Calcium Carbonate Hydrochloric Acid Carbonates Calcium Dietary Lithium Carbonate Antacids Calcium Citrate Nitric Acid Calcium Compounds Calcium Phosphates Aluminum Hydroxide.
What are the products when hydrochloric acid is added to calcium carbonate. Calcium carbonate reacts with hydrochloric acid in a double-replacement reaction. When calcium carbonate reacts with hydrochloric acid the products are calcium chloride carbon dioxide gas and water.
CaCO3 2 HCl CaCl2 CO2 H2O As the reaction happens in water CO2 will bubble out and the ions will be dissociated. As water forms the number of free. Calcium carbonate occurs naturally as chalk limestone and.
Ca2 2 Cl- If there is any doubt check on. Here is the unbalanced equation. Calcium carbonate reacts with hydrochloric acid to form calcium chloride water and carbon dioxide.
Calcium carbonate reacts with hydrochloric acid to form carbon dioxide gas. View the full answer. Calcium carbonate neutralizes stomach acid which is primarily hydrochloric acid by reacting with it to form carbon dioxide calcium chloride and water.
This is an acid-base reaction neutralization. Hydrochloric acid reacts with calcium carbonate to produce calcium chloride carbon dioxide and water. Look at the following examples.
Calcium carbonate reacts with hydrochloric acid to produce calcium chloride carbon dioxide and water. Answer 1 of 3. Calcium carbonate reacts with hydrochloric.
The commonest carbonate-acid reaction you will come across is that between calcium carbonate and dilute hydrochloric acid. CaCO_3 HCl -. Rate of reaction between Hydrochloric Acid and Calcium Carbonate.

Caco3 Hcl Calcium Carbonate Hydrochloric Acid Youtube
What Is The Reaction Between Calcium Carbonate And Hydrochloric Acid Quora

A Substitute Formulae For Names And Balance The Following Equation Calcium Carbonate Reacts Youtube
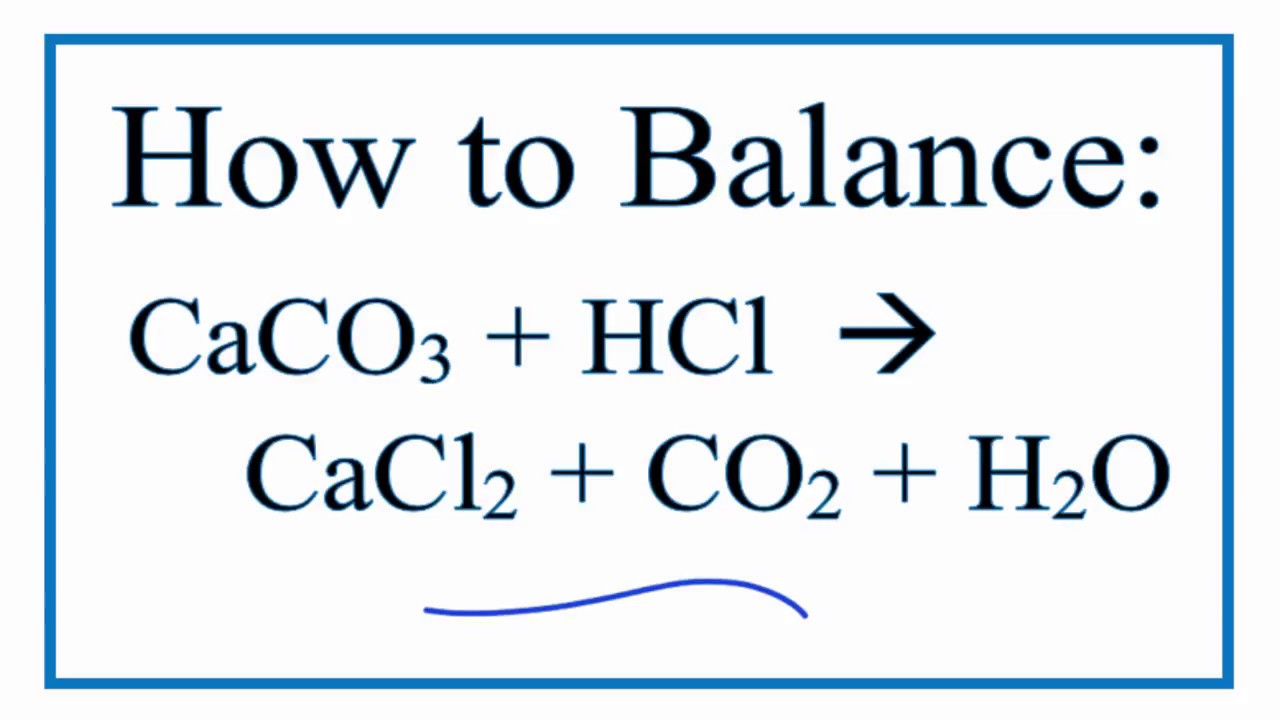
How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube
No comments for "Calcium Carbonate Reacts With Hydrochloric Acid"
Post a Comment